Spectacular Tips About How To Tell If A Molecule Is Planar

Anthracene (cyclic, planar) 7 π bonds.
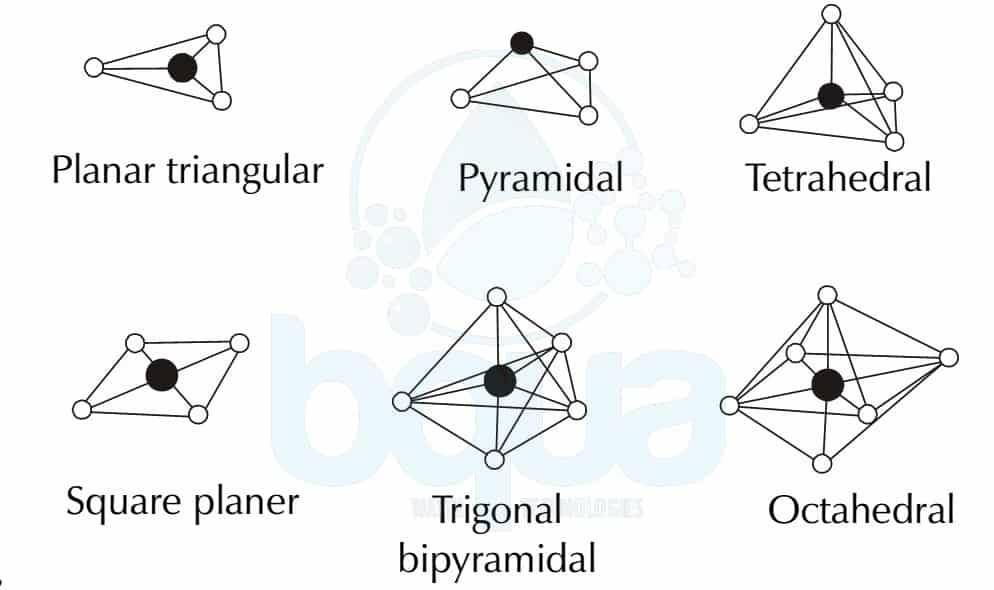
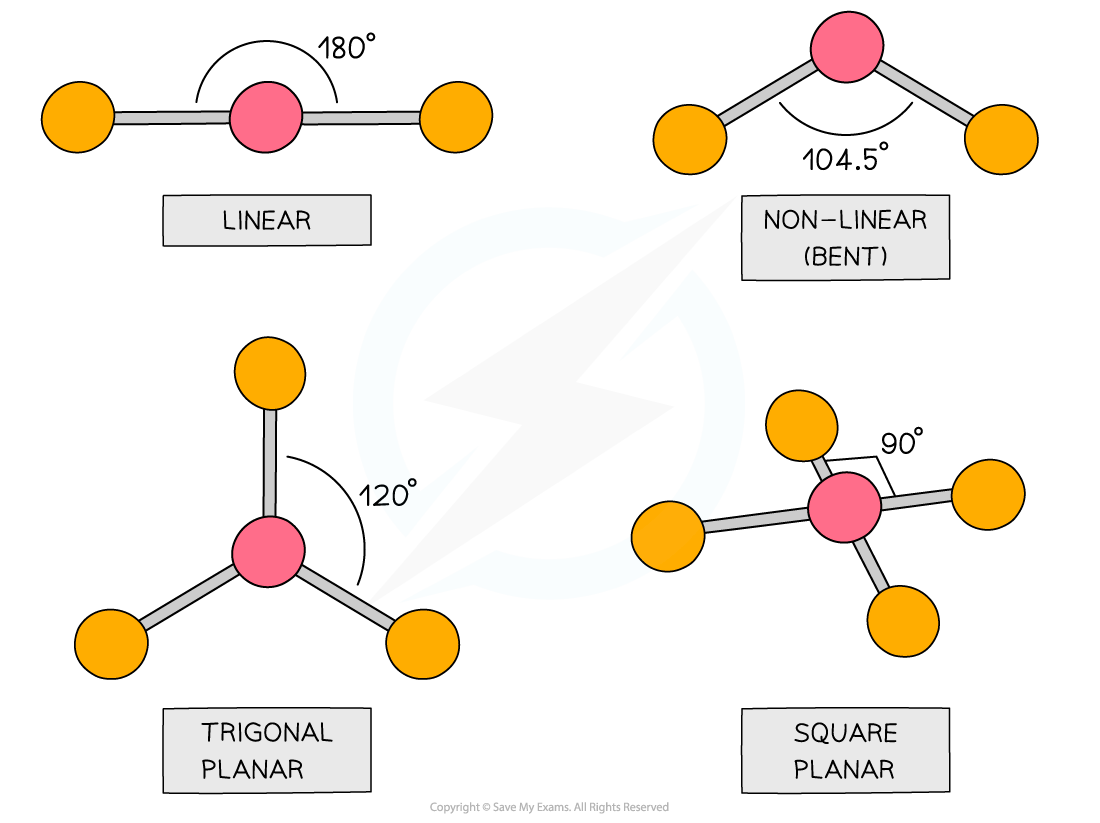
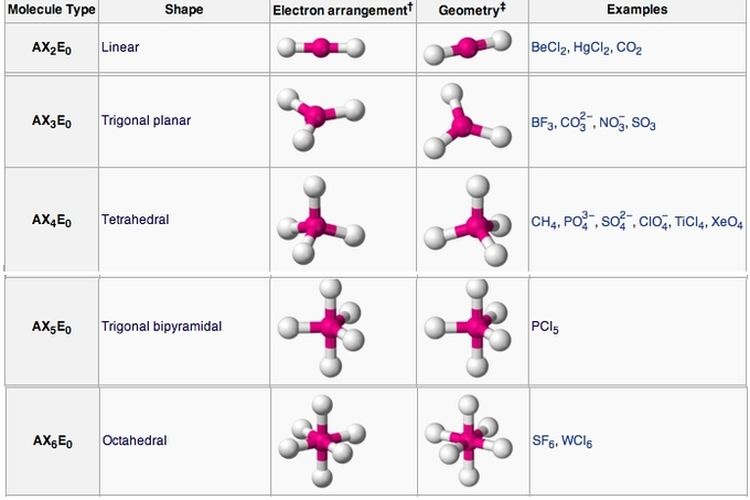
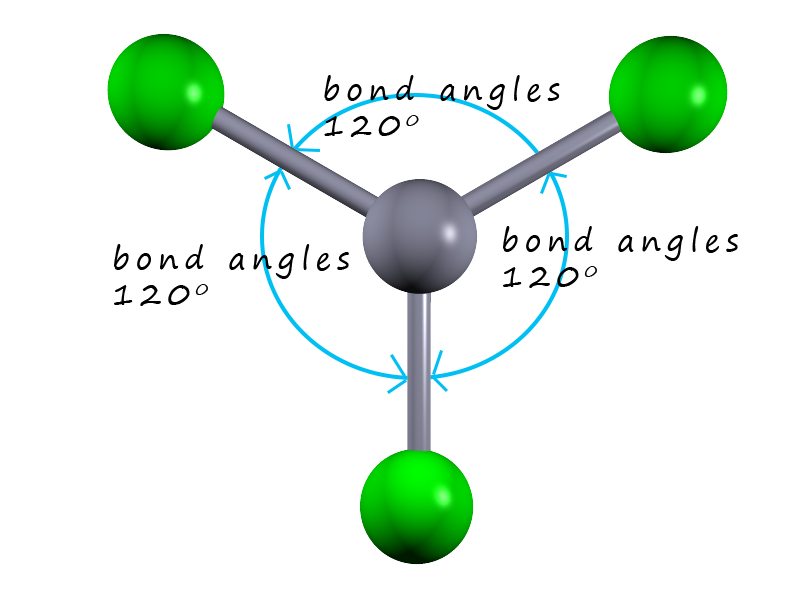
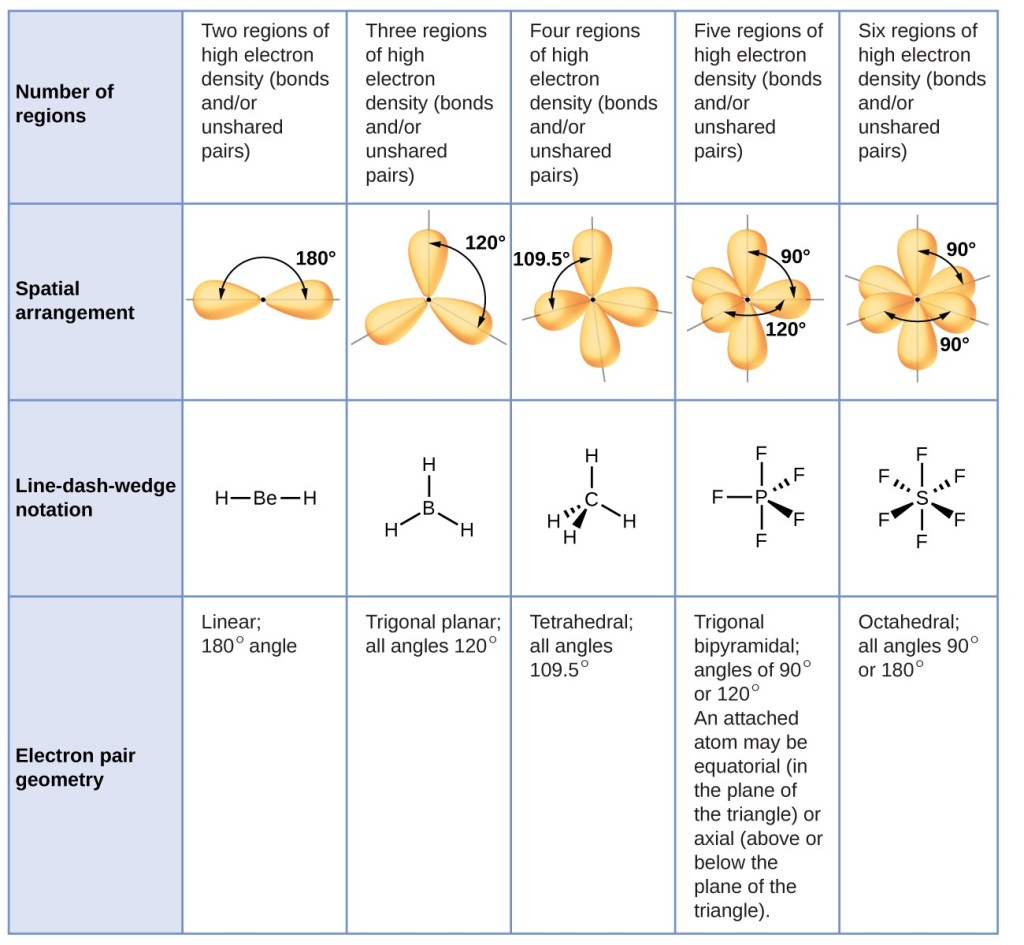
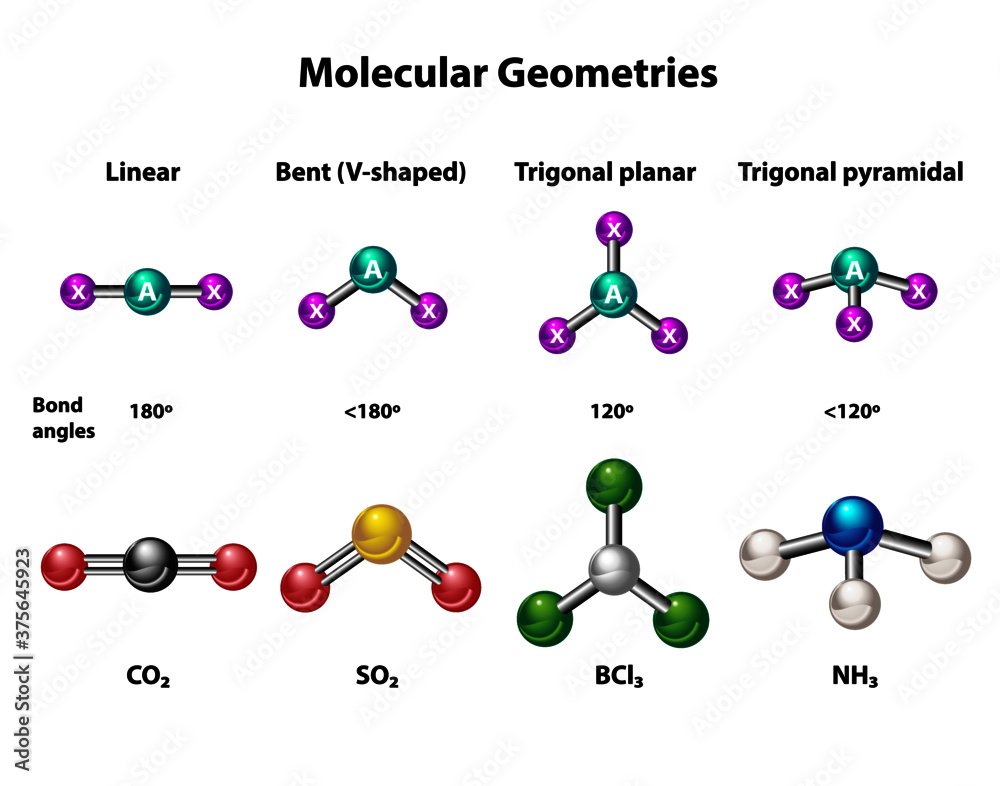
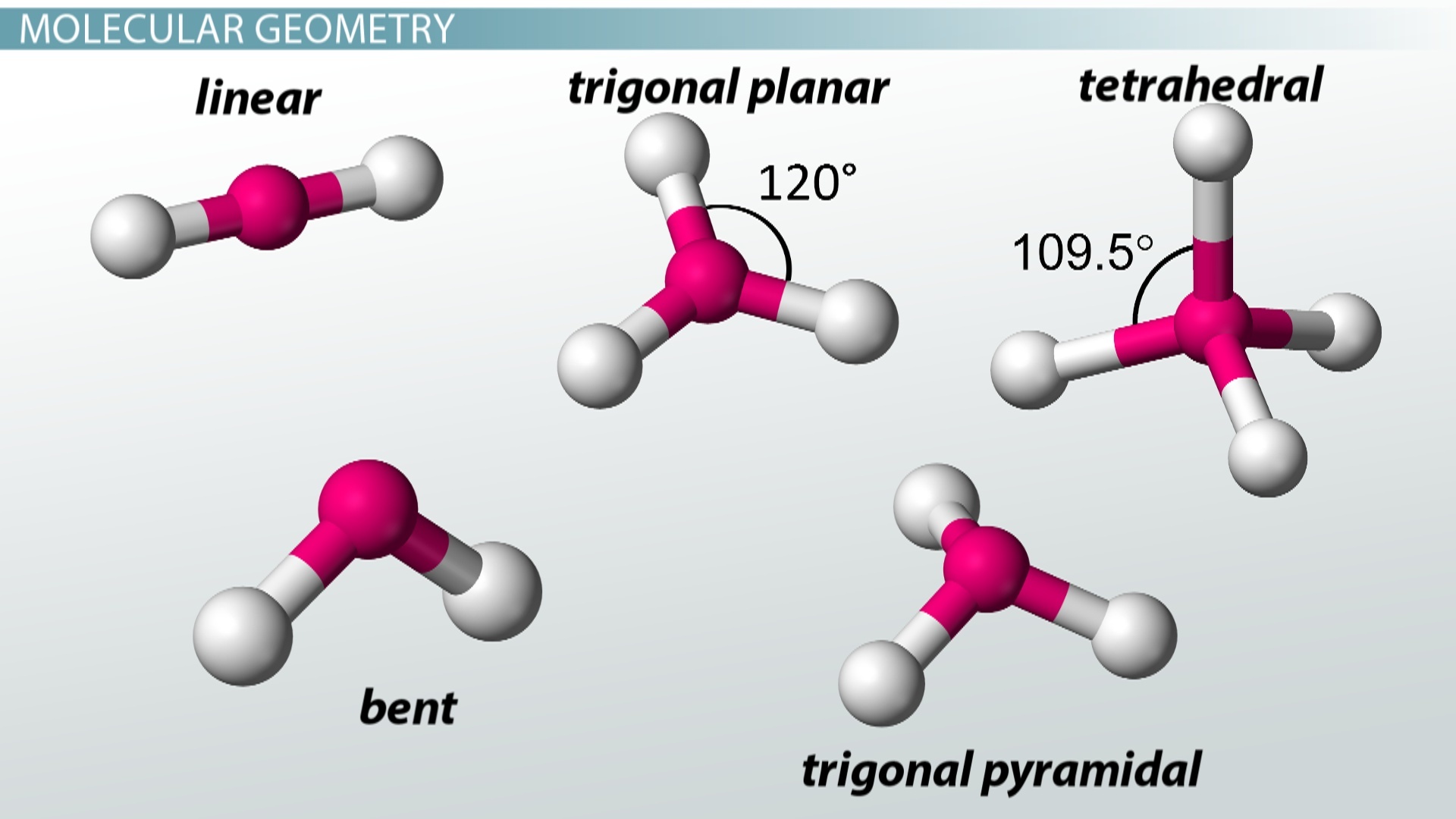
How to tell if a molecule is planar. If a molecule has 3 bonds and 1 lone pair, it is bent or angular. The three equatorial positions are separated by 120°. If a molecule has 4 bonds and 0 lone pairs, it is.
Another non polar molecule shown below is boron trifluoride, bf 3. How to determine whether a compound is ionic or. 7 + 0 + 1 = 8 (even no) aromatic.
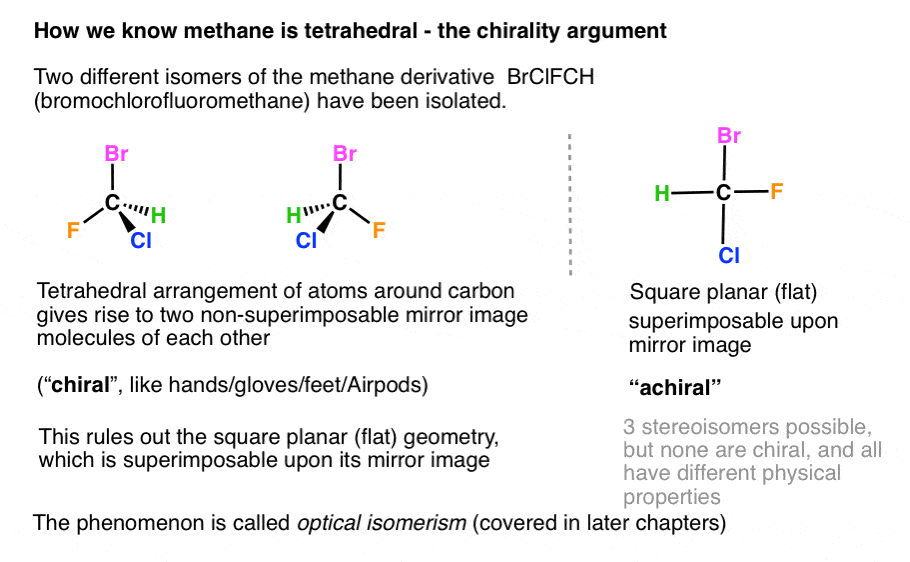
There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and bent with \(\approx \)120° bond angle (one lone pair). My idea was that for a compound to be planar the hybridization of all the atoms must be the. The answer is it adopts a geometry that is not planar (flat):
In sp² hybridization, one s orbital and two p orbitals hybridize to form three sp² orbitals, each consisting of 33% s. This means that the ring cannot contain a neutral sp 3 carbon. I was just reading about alpha and beta linkages in certain cyclic molecules, and it said that the alpha.
Square planar complexes. 5 + 0 +1 = 6 (even no) aromatic. Notice that a tetrahedral molecule such as \(\ce{ccl_4}\) is nonpolar figure (\(\pageindex{1}\).
Modified 3 years, 3 months ago. The presence of an sp 2 hybridised carbon can force the molecule to adopt a planar configuration (trigonal planar) the 3 σ bonds position themselves in a trigonal planar. If a molecule has 3 bonds and 0 lone pairs, it is trigonal planar.
How can one determine whether an organic compound is planar or not? When the cyclic organic molecule is planar in nature then the compound is said to be an aromatic compound. For a compound to be considered aromatic, it must be flat, cyclic, and conjugated and it must obey huckel's rule.
If there is an $\ce{sp^3}$ hybridized carbon (or nitrogen), the molecular is not planar. How to determine whether a compound is planar or not open in app. Huckel's rule states that an.
The double bonds are not in resonance as the p orbitals of adjacent double bonds cannot overlap. The molecule has three atoms in a plane in equatorial positions and two atoms above and below the plane in axial positions.